
Fertigation in acidic and alkaline soils
Fertigation in acidic and alkaline soils
Santosh Kumar Singh, Aradhna Kumari* and Ranjan Laik
Introduction
Fertigation is the injection of fertilizers, used for soil amendments, water amendments and other water-soluble products into an irrigation system. Fertigation is practiced extensively in commercial agriculture and horticulture. Fertigation is also increasingly being used for landscaping as dispenser units become more reliable and easier to use. Fertigation is used to add additional nutrients or to correct nutrient deficiencies detected in plant tissue analysis. It is usually practiced on high-value crops such as vegetables, turf, fruit trees, and ornamentals.
The benefits of fertigation methods over conventional fertilizing methods include increased nutrient absorption by plants, accurate placement of nutrients, where the water goes the nutrient goes as well, reduction of fertilizer, chemicals, and water needed, reduced leaching of chemicals into the water supply, reduced water consumption due to the plant’s increased root mass’s ability to trap and hold water.
However, there may be some disadvantages of it, like: Concentration of the solution may decrease as the fertilizer dissolves. The water supply for fertigation is to be kept separate from the domestic water supply to avoid contamination. Pressure loss is possible in the main irrigation line. For acidic and alkaline soils fertigation strategies vary, which are necessary to know. In this article, we will discuss in detail about fertigation of major, minor and trace elements in acidic and alkaline soils.
Acid soils
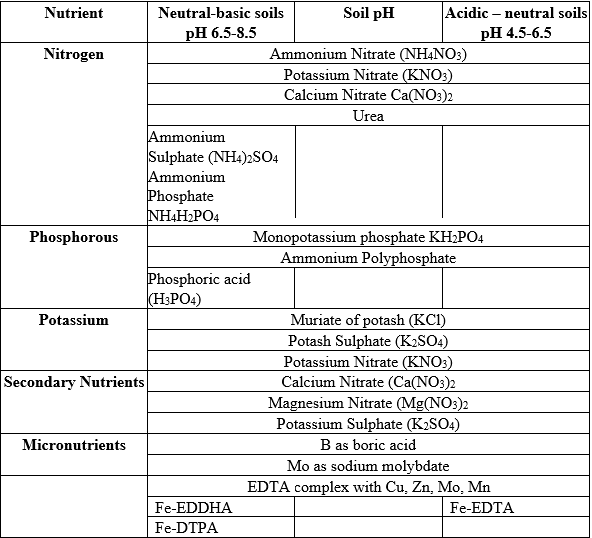
Acid soils are characterized by active aluminium (Al) ions, shortage of Ca, slow nitrification rate, and strong fixation of additional P from fertilizers. The use of nitrate fertilizers as N source, as suggested in Table 2, increases the pH in the rhizosphere due to nitrate nutrition. The increase of the pH in the rhizosphere alleviates Al ions toxicity and allows root elongation.
Alkaline soils
The characteristics of basic or alkaline soils are the presence of active Ca-carbonate, excess of soluble Ca ions, a rapid nitrification rate, and mild fixation of additional P from fertilizers. All types of N fertilizers are suitable to be added with the irrigation water. Even urea, which is completely soluble and causes an initial increase in pH due to the activity of urease in the soil, is safe to use in trickle irrigation as no local increase in urea concentration is expected in the soil. In alkaline soils, the clays are mainly of the 2:1 type, and ammonium is adsorbed to the clay and does not cause ammonium toxicity to root since it is diluted by the irrigation water. The same reasoning applies to all ammonium-based fertilizers.
The soil pH has no influence on any priority selection for K, secondary nutrients and all the micronutrients that are supplied in chelated forms, except for Fe2+. Since Fe-EDTA is not stable above pH 6.5 in basic soil, Fe-DTPA is recommended for soils with a pH up to 7.5, while Fe-EDDHA is recommended in extremely high pH soils since it is stable up to pH 9.
Table 1: Recommended fertilizers for fertigation in neutral-alkaline (6.5-8.5) and acid (4.5- 6.5) soils
Nitrogen (N) in fertigation
Nitrogen forms in fertilizers
There are three basic forms of nitrogen fertilizers
Urea–N: an electrically neutral molecule – CO(NH2)2.
Ammonium–N: which carries a positive electric charge – NH4 + cation. Nitrate–N: which carries a negative electric charge – NO3– anion.
In fertigation, applied urea travels with the water in the soil. The fertigated urea on reaching the boundaries of the wet zone becomes susceptible to volatilization. Evaporation from the soil surface results in increased urea concentration near the soil surface. This residual urea at the soil surface is also certain to be lost to the atmosphere as ammonia. When either ammonium or urea is used as a nitrogen source in fertigation, significant gaseous losses as N2O and nitric oxide (NO) have also been recorded. Another concern about urea is the potential problem related to the harmful effects of biuret, an impurity normally found at low concentrations. During germination and early growth of seedlings, biuret levels of up to 2% can be tolerated in most fertilizer programs recorded.
Ammonium (NH4+) carries a positive electric charge (cation) and is adsorbed to the negatively charged sites on clay and can also replace other adsorbed cations on the clay surfaces. These are mainly Ca and Mg that constitute the major sorbed cations in the soil. As a result of these interactions, ammonium is concentrated near the trickle and the displaced Ca and to a lesser extent Mg travels with the advancing water. Within a few days, the soil ammonium is usually oxidized by soil bacteria to the nitrate form that is dispersed in the soil with further irrigation cycles.
Nitrate (NO3 -) carries a negative electric charge (anion). It cannot, therefore, bind to the clay particles of basic and neutral soils which carry negative charges. However, nitrate binds to positively charged iron and aluminium oxides present in acid soils. As in the case of urea, nitrate travels with the water, and its distribution in the soil depends on the timing of its injection to the irrigation line. Nitrate is a strong oxidizing agent. Under the trickle, there is usually a certain soil volume that is saturated with water and, therefore, lacks oxygen (anaerobic conditions). Under such conditions, many soil microorganisms use the oxygen from the nitrate ion instead of molecular oxygen for their respiratory needs, resulting in the loss of nitrous oxide and dinitrogen gases to the atmosphere. This mechanism, the biological reduction of nitrate to nitrous oxide or dinitrogen (usually termed as “denitrification”) is responsible for some losses of N applied.
Phosphorus (P) in fertigation
Phosphorus (P) in solution is subject to interactions with inorganic and organic constituents in the soil. The H2PO4 – ion remains stable in the solution inside the irrigation line as long as the pH is kept low. Once it is released to the soil it reacts very quickly with clay minerals like, montmorillonite and illite in basic soils, and with kaolinite clay, iron and aluminum compounds in acid soils. P reacts mainly with lime (CaCO3) in basic soil conditions. The range of relatively insoluble chemical products of P with soil constituents is so large that it is generally called “fixed P.” The rapid reactions of phosphate with Ca (lime-rich soils) in basic soils, and with Fe and Al in acid soils restrict the distance of movement of applied P in the soil. The higher the clay content or CaCO3 fraction of the soil, the shorter is the distance of movement of P from the dripper. Even in sandy soils, the distance traveled by P is quite limited as compared with the water.
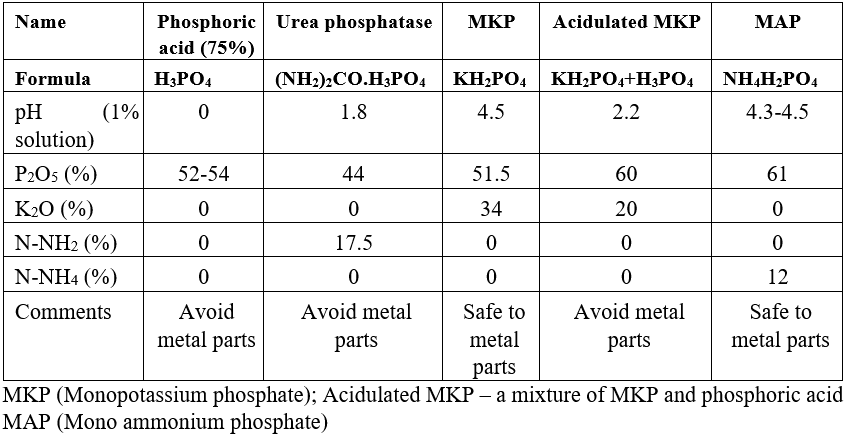
Table:2 Characteristics of P fertilizers used in fertigation
Phosphoric acid
In fertigation, phosphoric acid is used to clean fertigation lines from inorganic precipitates as well as opening clogs in drippers, and at the same time supplying P to growing plants. As phosphoric acid is a concentrated acid, care in handling should still be taken, such as wearing of goggles and gloves to protect from spills on skin and clothing. Since it is a highly concentrated P source, a separate delivery pump is used in the field.
Polyphosphate fertilizers
The term “poly” means that the molecular structure of the substance contains more than one P atom. Compounds having only one P atom are termed “orthophosphates.” By heating and removing the water molecule, a P molecule containing two P atoms is produced and is termed “pyrophosphate”, and when three and more P atoms are present in the molecule, the term used is “polyphosphate”. Pyrophosphate is the main form of condensed P in the liquid fertilizer ammonium polyphosphate (APP). When APP is applied to the soil, the pyrophosphate is hydrolyzed to orthophosphate. The only form of P taken up by the plant is the anion H2PO4 -, which means that the polyphosphate fertilizer must revert to the mono P form before the plant can take it up. This reaction needs an acidic environment for the supply of H+ ions (protons). The major supplier of protons is the root itself, which releases H+ ions into the soil solution during the uptake of ammonium-N. This production of H+ ions decomposes the polyphosphate and reverts it to mono-phosphate, which is available to the plant. In calcareous soil, the time needed to degrade 50% of the P (half-life) was found to be 14-21 days. This halflife is very long since about five half-life periods (i.e. 70-100 days) are needed for 90% of the material to revert to plant-available forms.
Urea Phosphate (UP) (CO(NH2)2∙H3PO4)
Urea phosphate is a chemical adduct between urea and phosphoric acid molecules. It contains a minimum of 17.5% N and 44% P2O5. It is used in the fertigation of soil-grown crops under neutral and alkaline conditions. After dissolution, 6.3 mol H+ per kg UP is released, which makes it a concentrated acidifier. Due to its acidic action, it helps to keep tank solutions clear and prevent clogging of the fertigation equipment. Urea phosphate reduces the pH of the irrigation water and soil, which improves nutrient availability and nutrient uptake efficiency. In calcareous sodic soils, UP reacts with calcium carbonate, the calcium ion replacing sodium from the soil complex, which improves the soil structure (less compaction). After flushing with sufficient water, sodium is washed out of the rooting zone. As a result, water infiltration is improved, and sodium levels in the rooting zone are reduced. The risk of N volatilization is reduced with UP.
Monopotassium phosphate (MKP) (KH2PO4)
Monopotassium phosphate is a soluble salt of potassium hydroxide and phosphoric acid. It contains 51.5% P2O5 and 34% K2O. It is used in fertigation when the daily supply of P is recommended and in sand dune cultures. Due to its very low salt residues, it is especially suitable in saline water open-field agriculture.
Acidulated MKP (KH2PO4 + H3PO4)
This is a new fertilizer introduced recently to increase the P concentration to 60% P2O5 and to increase acidity to prevent P precipitation and clogging of the irrigation lines when hard water (high Ca content) is the irrigation water source.
Mono ammonium phosphate (MAP) (NH4H2PO4)
Mono ammonium phosphate fertilizer contains 61% P2O5 and 12% N in the ammonium form. It is commonly used in fertigation field practices.
Potassium (K) in fertigation
There are four K fertilizers available for fertigation: potassium chloride (KCl) or muriate of potash (MOP), potassium sulfate (SOP), monopotassium phosphate (MKP) and potassium nitrate (KNO3).
Potassium chloride is the most abundant K fertilizer in the world. It is soluble, dissolves quickly and is easily mixed with other N fertilizers. Reasons against its use usually point to the accompanying chloride (Cl–) anion. The amounts of Cl supplied which Cl interferes with the burning quality. In most other crops, KCl is an acceptable fertilizer.
Potassium sulfate, K2SO4, is widely used under saline conditions. Due to the presence of SO4, it is used when the water available is low in Ca, i.e. only when “soft” water is available for irrigation. The presence of high Ca in the water result in gypsum precipitation in the irrigation lines, clogging the drippers.
Monopotassium phosphate can be a source of K, but it is more of a source for P in fertigation. Since the amounts of P needed by plants are only about 1/10 of the amount of K uptake, this fertilizer is considered mainly as a P source in fertigation.
Potassium nitrate is highly soluble in temperatures above 20 ºC and presents an optimum ratio of K: N from a nutrient uptake viewpoint. At low night temperatures, this fertilizer can precipitate in the tank so special care must be taken when open field storage of KNO3 containers are left overnight.
Secondary nutrients in fertigation
Calcium
Calcium is unique in its behaviour in the plant. It should be continuously supplied in the soil solution to the elongating roots. It moves in the plant one way only from the root to the top, and it is the only element that does not move back in the phloem from the leaves to the roots or the developing fruits. Therefore, any shortage of Ca supply to the roots results in root cell death in the elongation zone. This is the main reason for restricted root growth in acid soils and the reason for adding calcium carbonate (CaCO3) or limestone to lower soil acidity and promote root proliferation.
In fertigation, calcium nitrate 5(Ca(NO3)2∙2H2O)∙NH4NO3 is the main source of Ca. This fertilizer is essential when irrigation water is very low in Ca. In dry land and highly carbonate-rich soil, additions of Ca in fertigation should be carefully checked since highly Carich water may clog the emitter by CaCO3 precipitation if leftovers are not flashed at the end of the irrigation.
Magnesium (Mg)
Mg fertilizers are available in several forms:
- Kieserite (MgSO4∙H2O), a naturally occurring mineral and used as a soluble Mg fertilizer in acid soils, dolomitic limestone, calcined magnesite, and fused magnesium phosphate.
- Soluble Mg fertilizers: magnesium nitrate (Mg(NO3)2∙6H2O) and magnesium sulfate (MgSO4∙7H2O) are used mainly in highly soluble formulations in fertigation practice.
Sulfur (S)
Sulfur is an essential element and is present in the plant in quantities close to those of P. Being an essential element, its supply (as sulfate ion SO42-) in the irrigation water usually meets plant requirements. Its presence as the anion in potassium sulphate, ammonium sulfate and Mg fertilizers is able to supply all the plant’s needs for S.
Micronutrients in fertigation
In fertigation, most of the metal micronutrients such as Cu, Fe, Mn, and Zn, are supplied in a chelated form, mainly as EDTA [ethylene-diamine-tetraacetic acid]. In this form, most of the metal-chelate compounds are stable below pH 7.0. Stable Fe chelates for alkaline soils (pH>7.5) are usually EDDHA [ethylene-diamine di ortho-hydroxyphenyl acetic acid] based. Iron chelate compounds are indispensable to fertigation. High levels of plant-available Cu above a few g m-3 can cause toxicity effects known as ‘copper shock’. Thus, careful control of Cu levels in fertigation solutions is essential.
In a pure fertigation solution, B is present as boric acid [B(OH)3] or as the borate anion [B(OH)4-]. The pH around the root affects B uptake. Above pH 8 there is a marked and fast decline in B uptake. Molybdenum is usually not included in any fertilizer formulas unless a specific demand is identified due to plant deficiency symptoms.

